Background
Mesenchymal stromal cells (MSC) are vital components of the bone marrow (BM) microenvironment niche and regulate hematopoietic stem and progenitor cell (HSPC) activity. While HSPC harbor primary disease driver mutations, MSC in myelodysplastic syndrome and myeloproliferative neoplasm (MDS/MPN) have been shown to influence the emergence and selection of leukemic clones and disease progression. However, it remains unclear if there are specific functional characteristics or genetic signals of MSC that interact with HSPC at different stages of disease progression. We examined BM samples from MDS/MPN patients with high rate of leukemic transformation in less than 2 years (HR) versus those with stable disease for 5 years (SD), to investigate the phenotypic differentiation, functional characteristics, and transcriptomic profiles of MSC and the HSPC counterparts.
Methods
We obtained BM samples at diagnosis and follow-up from MDS/MPN patients including chronic myelomonocytic leukemia (CMML) treated at Singapore General Hospital. We studied 5 HR and 13 SD patient samples to compare against 5 healthy donor samples. MSC were derived in MesenCult-ACF media (StemCell Technologies), and phenotypic characterization was done after 1-2 passages. HSPC were subjected to co-culture expansion with MSC in MyeloCult-H5100 for 7 days, and long-term culture-initiating cell assays (LTC-IC) using MSC as feeder cells for 6 weeks. Total RNA extracted from MSC and HSPC were subjected to bulk and single-cell RNA sequencing (scRNA-Seq) (10X Genomics platform). Principal component analysis (PCA) was used for the uniform manifold approximation and projection (UMAP). Gene set enrichment analysis (GSEA) was performed on differentially expressed genes to examine canonical signaling pathways. Cellchat algorithm was applied to predict intercellular communication between MSC and HSPC using scRNA-Seq data.
Results
Baseline clinical variables for the international prognostic scoring system (IPSS) including age at diagnosis, myeloblast percentage and cytogenetics were not significantly different between HR and SD patients. Decreased osteogenic and chondrogenic differentiation, but relatively preserved adipogenic differentiation capacity was observed in all MDS/MPN MSC samples. A significantly lower yield of CD34+ CD38- primitive HSPC with myeloid skewing was observed in the co-culture experiments with MDS/MPN MSC, compared to healthy MSC. Pre-treatment of SD-MSC feeder with the hypomethylating agent 5-azacytidine improved the clonogenic potential of the corresponding co-cultured HSPC, but did not restore the HSPC colony yield with HR-MSC feeder.
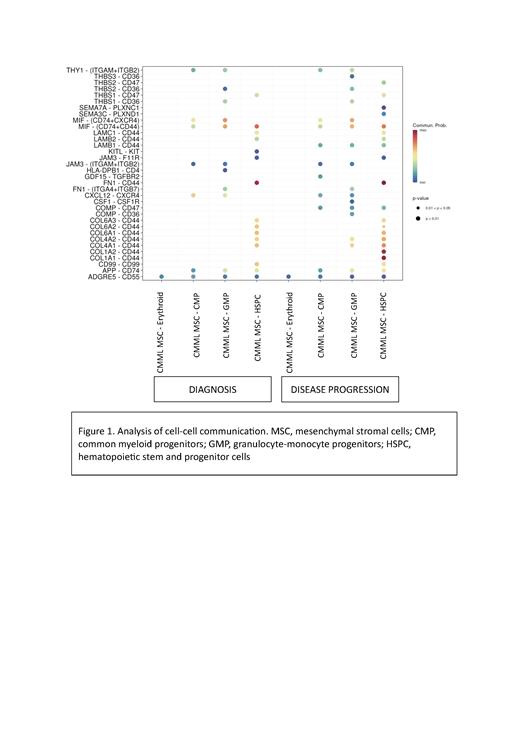
Unbiased PCA of bulk RNA-Seq data demonstrated greater clustering of HR-MSC, while SD and healthy MSC overlapped significantly. Genes associated with mesenchymal multipotency and differentiation (PODXL20, FOXQ122, SERPINA923) were generally downregulated in MDS/MPN MSC, likely attributed to promoter hypermethylation. Significant differential upregulation of CD74 and IL-6 expression was observed in HR-MSC, which suggested a uniquely enriched inflammatory signature. GSEA results showed that pathways associated with G2M cell cycle checkpoint, DNA repair, glycolysis, and response to azacytidine were suppressed in HR-MSC. UMAP on scRNA-Seq data of longitudinal MSC samples identified distinct clustering of HR-MSC at disease progression. Cell-cell interaction analysis predicted a strong communication between collagen-encoding genes (COL1A1 and COL1A2) in HR-MSC at diagnosis and CD44, a negative regulator of HSPC proliferation, and the interaction was further enhanced at disease progression (Figure 1). Ongoing analysis of downstream gene expression changes and secretome profile of HSPC in MDS/MPN MSC-based in-vivo scaffold models will be presented.
Conclusion
Together, our data point towards a degree of co-development and communication between MSC and HSPC during disease progression of MDS/MPN. Further characterization of the unique functional and transcriptomic signatures in MSC may help to optimize disease prognostication and identify novel therapeutic targets in MDS/MPN patients.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal